Lines Spectra and Excited Electron States
$ 14.50 · 4.8 (518) · In stock
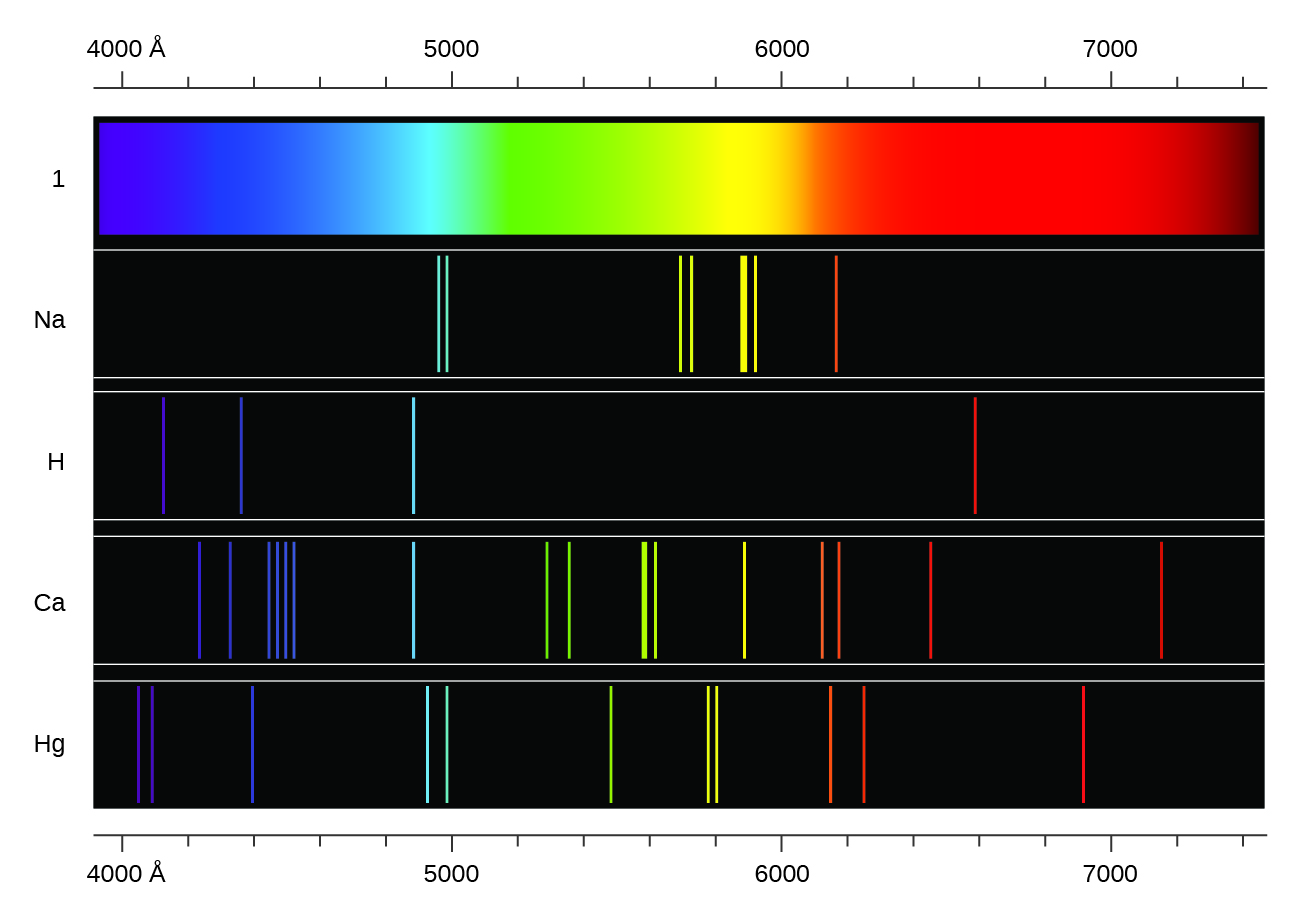

2.2 Hydrogen emission spectrum (SL)
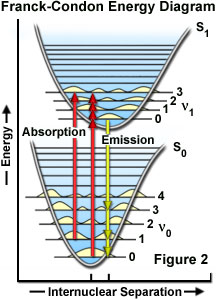
Confocal Microscopy - Fluorescence Excitation and Emission Fundamentals
maximum number of spectral lines obtained in balmer series when electron from fourth excited state falls to the ground state in hydrogen atom i

Excited state spectroscopy (kT ≈ 3.4 µeV), showing that the feedback

Spectroscopy 101 – How Absorption and Emission Spectra Work

Machine learning lets researchers see beyond the spectrum
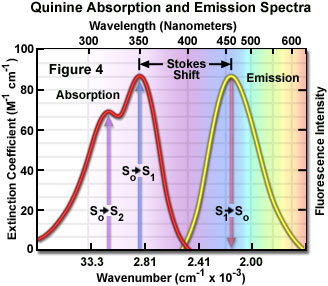
Confocal Microscopy - Fluorescence Excitation and Emission Fundamentals
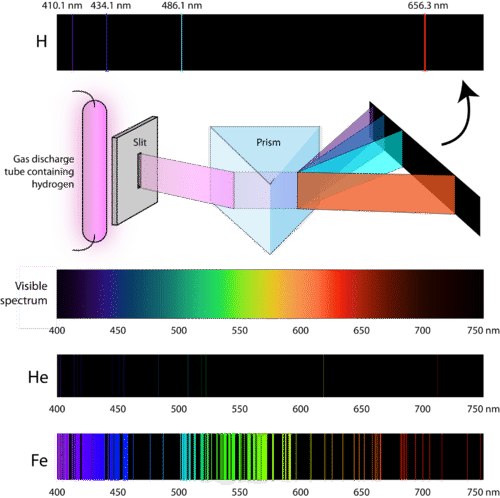
Atomic Emission Spectra

Formation of Spectral Lines