Why are atomic spectra of an element discontinuous?
$ 19.50 · 4.9 (646) · In stock

22.1 The Structure of the Atom
Question #27e1c
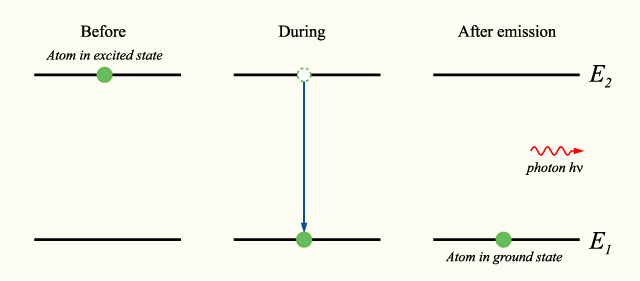
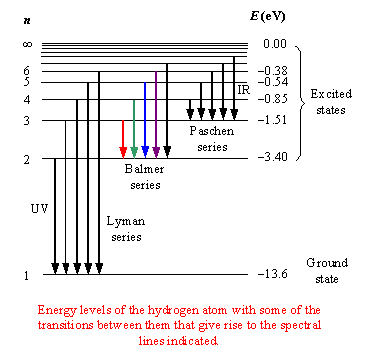
When an electron jumps from a high energy state to a lower state, what form does the emitted energy take?
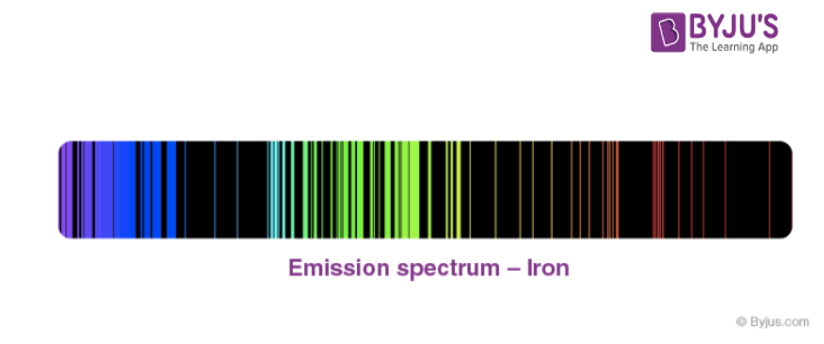
Atomic Spectra (Emission Spectrum & Absorption Spectra) - Detailed Explanation with Videos
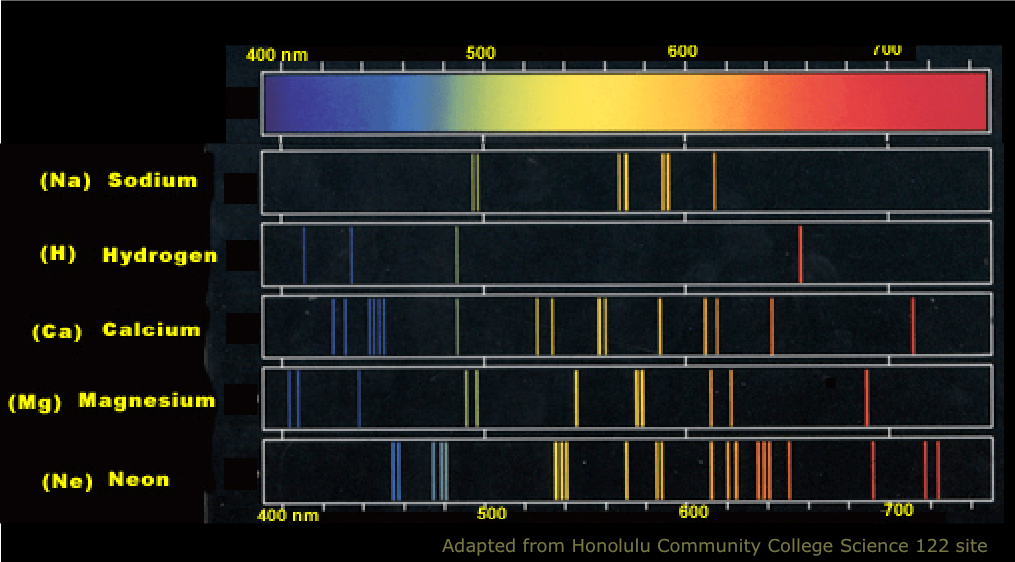
How do colored spectral emission lines relate to frequency?

Why are atomic spectra of an element discontinuous?

Distinguish between continuous spectrum and discontinuous sp

Find out the incorrect match. (1) Electron - Dual nature (2) Rainbow - Discontinuous spectra (3) Atoms Line spectra (4) Particle nature - Photoelectric effect According to the Rohr Thean, whicl -01
How can atomic emission spectra be useful?

Difference Between Continuous and Discrete Spectrum

Why Are Atomic Emission Spectra Discontinuous?
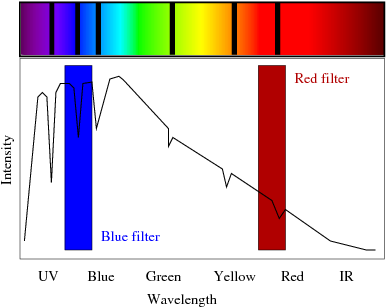
Absorption and Emission

SOLUTION: 10 light and quantized energy atomic emission spectra 1 - Studypool
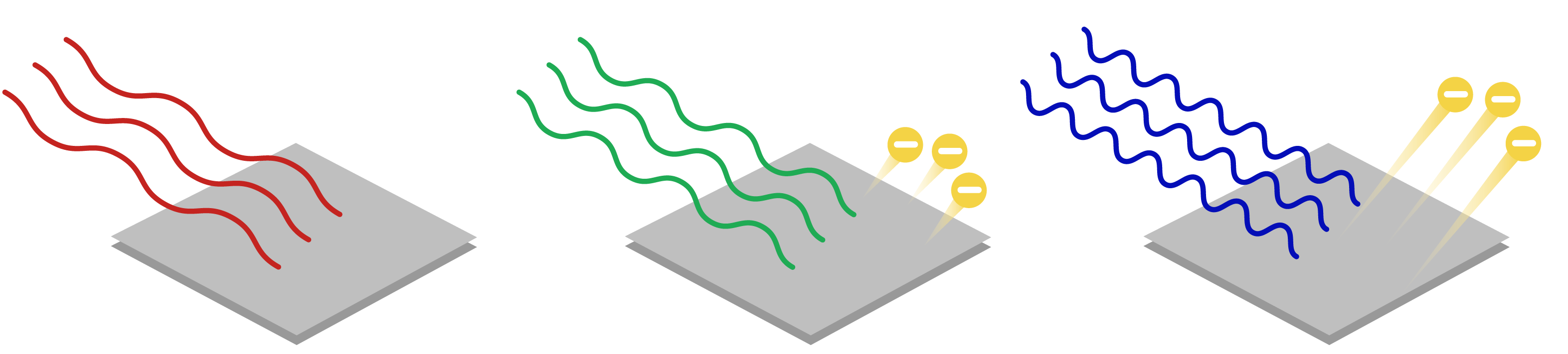
Question #79130
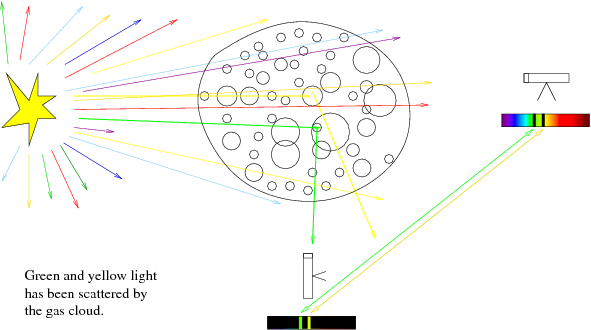
Absorption and Emission